
Print ISSN: 2581-5725
Online ISSN: 2456-9267
CODEN : IACHCL
IP Archives of Cytology and Histopathology Research (ACHR) open access, peer-reviewed quarterly journal publishing since 2016 and is published under the Khyati Education and Research Foundation (KERF), is registered as a non-profit society (under the society registration act, 1860), Government of India with the vision of various accredited vocational courses in healthcare, education, paramedical, yoga, publication, teaching and research activity, with the aim of faster and better dissemination of knowledge, we will be publishing the article more...Null
Author Details :
Volume : 4, Issue : 1, Year : 2019
Article Page : 70-74
https://doi.org/10.18231/2456-9267.2019.0012
Abstract
Introduction: Urinary bladder cancer is common malignancy of the genitourinary tract in both males and females.CD10 is cell surface zinc dependent metalloprotease which may play a crucial role in tumor progression, invasion and metastasis. The present study was done to analyze staining pattern and intensity of CD10 and its association with grade, stage and prognosis of urothelial carcinoma.
Materials and Methods: The prospective study included 51 cases of urothelial carcinoma over a period of one year which were followed up for three years. The staining pattern and intensity of CD10 immunostaining was correlated with histopathological grade, stage and survival of these patients.
Results: The maximum number of urothelial carcinoma cases (33.3%) were in the age group of 60-70 years with mean age of 60.4 years. The staining pattern and intensity of CD10 showed statistical significant increase with increase of histopathological grade, stage of tumor and with decrease of survival of patients (p<0>
Conclusion: The study concludes that there is significant association between staining intensity and score of CD10 immuno-expression with histopathological grade, stage and the survival in patients of urothelial carcinoma.The staining intensity and pattern of CD10 immuno-expression may be helpful in determination of prognosis of urothelial carcinoma cases. In addition, it is suggested that further larger studies with extended follow ups may be done to evaluate the exact role of CD10 in prognosis of urothelial carcinoma.
Keywords: Urothelial carcinoma, CD10, Histopathological grade, Stage, Survival, Prognosis.
Urinary bladder cancer represents the common and frequent malignancy of the genitourinary tract in both males and females. Globally the incidence of bladder cancer is 3.1% with the mortality of about 2% and age standardized rate is of 5.3/100000 population.[1]In India the incidence of bladder cancer is 1.6% with the mortality of about 1.4% and age standardized rate of 1.6/100000 population.[1] Approximately 90% of malignant urinary bladder cancers are transitional cell carcinoma (TCC) with highest incidence in the sixth and seventh decades of life with men affected more than women.[2]
CD10 is also known as common acute lymphocytic leukemia antigen (CALLA). It is 90-110 kDa, a single-chain antigenic molecular marker which is a cell surface zinc dependent metalloprotease that inactivates bioactive neuropeptides and limits its cellular responses to peptide hormones by hydrolyzing them. CD10 may play a crucial role in tumor progression, invasion and metastasis in colorectal carcinomas, breast carcinomas, malignant melanomas and stromal tumors.[3],[4],[5] It has also an inhibitory effect on proliferation and progression of tumor cells in prostatic carcinomas and lung carcinoma usually by stimulating apoptosis.[6],[7]
Nowadays, many clinical, biochemical, histopathological, immunological and cytogenetics features have been recognized as variables for assessing the risk groups for urothelial carcinoma patients and to determine the recurrence of tumor and survival among patients after radical cystectomy. The histopathological features include histopathological grade, TNM stage which depends on the depth of muscle invasion, degree of tumor differentiation, size of growth, presence of local and distant lymph node metastases specially for recurrenceof the growth.[8] To date, few studies have investigated the staining intensity and pattern of CD10 expression in urinary bladder but a systematical analysis of staining pattern and intensity of CD10 with prognostic significance in urothelial carcinoma is lacking. Therefore, the present study was done to analyze staining pattern and intensity of CD10 and its association with grade, stage and prognosis of urothelial carcinoma.
The cross-sectional study was conducted in the Department of Pathology at an institute which including a total of 51 consecutive cases of urothelial carcinoma over a period of one year after obtaining written informed consent. All the relevant clinical details of history, physical examination and investigations were recorded in the case reporting form. The histopathological grading and staging of urothelial carcinoma was done according to the WHO histological classification of urothelial carcinoma. Immunohistochemical analysis for CD 10 was performed on paraffin embedded, formalin fixed biopsy sections as per standard procedure (BioGenex, CA, USA). The extent of immunoreactivity to CD10 was scored semiquantitatively based on the percentage of positive cells and were classified on a three point scale.[6]Score 0(<5>+ 2 (strong expression; >50% positive cells). For scoring of staining pattern and intensity, score 0 (no observable staining), Score 1 (incomplete membranous staining in ?10% of invasive tumor cells), Score 2 (both circumferential membranous and cytoplasmic staining in ?10% of invasive tumor cells, score 3 (homogeneous, dark, diffuse both cytoplasmic and nuclear staining >10% of invasive tumor cells). All the cases were followed up for a period of 36 months after diagnosis.
The data was entered on excel sheet and statistically analyzed using SSPS version 22. The expression of CD10 in urothelial carcinoma was compared with histo-pathological features of urothelial carcinoma by using Fischer Exact Test. Data analysis of correlation and association, if any, of CD10 expression in relation to histo-pathological features of urothelial carcinoma was done according to chi square test. The research and ethical committee of the institute approved the study.
The maximum number of urothelial carcinoma cases (n=17; 33.3%) were in the age group of 60-70 years, followed by 70-80 years (n=13; 25.5%). Mean age of the patients were 60.40±13.3 years with the range of 21 years to 78 years. 88% of the urothelial carcinoma cases (n=45) were males and 12% (n=6) were females with male to female ratio of 7:1. Out of total 51 cases, 78.4 % of the cases (n=40) showed high grade urothelial carcinoma while 21.6% of the cases (n=11) showed low grade urothelial carcinoma. Table 1 shows the staining pattern and intensity of CD10 for different histopathological grade of tumor. It shows statistical significantly greater score of CD10 staining pattern and intensity with increase of grade urothelial carcinoma. Table 2 shows the staining pattern and intensity of CD10 for different stage of tumor. It shows statistically significantly higher score of staining pattern and intensity of CD10 with increase in stage of urothelial carcinoma. Table 3 shows association of staining pattern and intensity of CD10 with 3 year survival of urothelial carcinoma patients.
Table 1: Staining pattern and intensity of CD10 with histo-pathological grade of tumor
|
Histopathological grade of tumor |
Staining pattern and intensity of CD10 |
Total(n) |
|||
|
Score 0 |
Score 1 |
Score 2 |
Score 3 |
||
|
High Grade UBC |
0 |
6 |
16 |
18 |
40 |
|
Low Grade UBC |
0 |
10 |
1 |
0 |
11 |
|
p value |
0.000 |
51 |
|||
Table 2: Staining pattern and intensity of CD10 with Stage of tumor
|
Histopathological Stage of tumor |
Staining pattern and intensity of CD10 |
Total(n) |
|||
|
Score 0 |
Score 1 |
Score 2 |
Score 3 |
||
|
Stage 1 |
0 |
11 |
5 |
3 |
19 |
|
Stage 2 |
0 |
5 |
8 |
7 |
20 |
|
Stage 3 |
0 |
0 |
3 |
6 |
09 |
|
Stage 4 |
0 |
0 |
1 |
2 |
03 |
|
p value |
0.021 |
51 |
|||
Table 3: Staining pattern and intensity of CD10 with living status
|
Living Status |
Staining pattern and intensity of CD10 |
Total (n) |
|||
|
Score 0 |
Score 1 |
Score 2 |
Score 3 |
||
|
Dead |
0 |
2 |
17 |
28 |
37 |
|
Alive |
0 |
14 |
0 |
0 |
14 |
|
p value |
0.000 |
51 |
|||
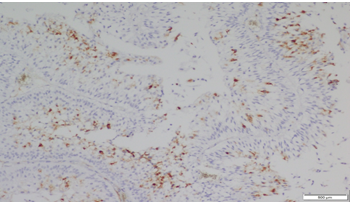 |
Click here to view |
Fig. 1: Staining pattern and intensity of CD10 of Score 1 in Low grade UBC (10x)
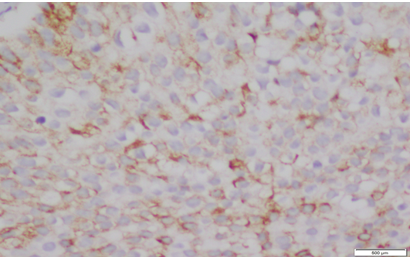 |
Click here to view |
Fig. 2: Staining pattern and intensity of CD10 of Score 2 in Low grade UBC (20x)
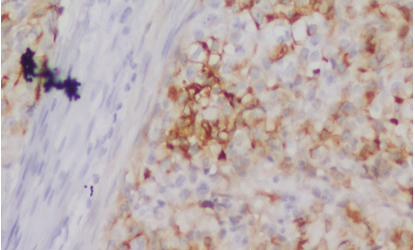 |
Click here to view |
Fig. 3: Staining pattern and intensity of CD10 of Score 1 in High grade UBC (20x)
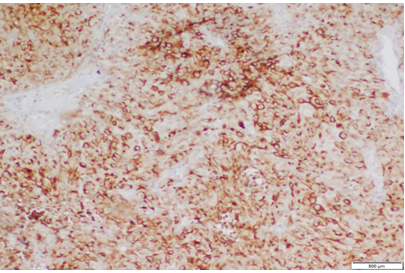 |
Click here to view |
Fig. 4: Staining pattern and intensity of CD10 of Score 2 in High grade UBC (10x)
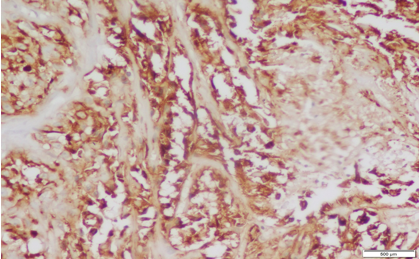 |
Click here to view |
Fig. 5: Staining pattern and intensity of CD10 of Score 3 in High grade UBC (20x)
In the present study total 51 cases of urothelial carcinoma and 50 non-neoplastic urinary bladder lesion cases were studied for one year. Urothelial carcinoma represents the second most common malignancy of the genitourinary tract in both males and females following prostate cancer in males. In the present study, urothelial carcinoma was more common in males with male to female ratio of 7:1. Previously, Mills SE et al and Prout GR et al have also observed that males were more commonly affected than females in urothelial carcinoma.[2],[9] However, in our study, the male: female ratio was much higher than observed by other previous studies. This might be associated by the fact that the males have more common habit of smoking than females in this region which may play significant role in carcinogenesis of the tumor. In our study, maximum number of urothelial carcinoma cases (n=17; 33.3%) were in the age group of 60-70 years with mean age of 60.40 years. The study also showed that low grade urothelial carcinoma was more common in sixth decade in comparison to high grade urothelial carcinoma which was more common in older age group (70- 80 years). The previous study conducted on 125 cases of urothelial carcinoma bladder (104 males and 21 females) by Rehmani et al showed that peak incidence was observed in the age group of 50-70 years with the mean age being 55 years.[10] Messinget al have also concluded that urothelial bladder carcinoma is generally a disease of middle-aged and elderly people with the median age of 70 years.[11]In our study maximum number of patients had high grade urothelial carcinoma (78.4%) and out of it 7.5% of cases showed squamous differentiation. All the three cases which showed squamous differentiation were of high grade and observed deep muscle invasion. Out of these, two cases died within a period of one year thus suggested that urothelial carcinoma with squamous differentiation is associated with higher grade, advance stage and poor prognosis. Similarly Lee et al concluded that squamous or glandular differentiation of urothelial carcinoma independently predicted poor patient
outcome.[12]The present study observed that 68.62% of total urothelial carcinoma cases (n=35) showed positivity for CD10 expression and out of it 51.42% (n=18) cases showed score +2 of CD10 immuno-expression. The study also showed that the intensity of CD10 immuno-expression and score of staining pattern increase with the grade of tumor. Chu et al also observed in their study that 54% of urothelial carcinoma cases were positive for CD10 staining while non-neoplastic tissue did not show any reaction for CD10. They concluded that malignant bladder epithelium rather than non malignant epithelium have a higher tendency for CD10 expression.[13] Langner et al confirmed the association of CD10 positivity with high stage of tumor in 53 cases of urothelial carcinoma.[14] Our study observed that only 4% of non-neoplastic urinary bladder lesion cases showed positivity for CD10 expression and this expression was of low score (score 1+). In contrast Murali et al observed that 50% of non-neoplastic uro-epithelium showed positivity for CD10 staining.[15]Kandemir et al suggested that CD10 expression in high grade urothelial carcinoma may be an outcome of genetic changes causing wild type or mutant type CD10 antigenic expression in the high grade carcinomas.[16]
The present study also observed that as the stage of tumor increases from stage 1 to stage 4, there was statistical significant increase in CD10 immuno-expression, staining intensity and score of staining pattern (p<0>et al conducted study on 53 cases of urothelial carcinoma and they confirmed the association of CD10 positivity with high stage and shorter disease free survival (5 year survival rate).[14]Another study also reported correlation of CD10 expression with the grade and stage of urothelial carcinoma and shorter mean survival.[15]In contrast Bircan et al detected a significant inverse correlation between pathological stage and CD10 immunoexpression.[17]The authors suggest that further larger studies should be done to correlate the staining pattern and intensity of CD10 with prognosis of urothelial arcinoma for definite comment on this association.
The study concludes that there is significant association between staining intensity and score of CD10 immuno-expression with histopathological grade, stage and the survival in patients of urothelial carcinoma.The staining intensity and pattern of CD10 immuno-expression may be helpful in determination of prognosis of urothelial carcinoma cases. In addition, it is suggested that further larger studies with extended follow ups may be done to evaluate the exact role of CD10 in prognosis of urothelial carcinoma.
Financial Support: None.
Conflicts of Interest: None.
How to cite : Shukla S K, Chandra S, Chauhan N, Rajeev R, Kusum A, Staining pattern and intensity of CD10 expression in Urothelial carcinoma and its prognostic significanc. IP Arch Cytol Histopathol Res 2019;4(1):70-74
This is an Open Access (OA) journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.