Introduction
Primary carcinoma of fallopian tube is uncommon and it has been considered to account only 0.7 - 1.5% of all gynecological malignancies.1 However the true frequency is difficult to determine, partly because some fallopian tube carcinoma might be misclassified as ovarian origin. Some ovarian serous carcinomas probably are of tubal origin. The tubal secretory cells may be considered as the initiation site of ovarian carcinogenesis according to the new ovarian carcinogenesis concept. 2 Primary tubal carcinoma has a wide range of age 25-95 years and vast majority are in post menopausal age group. 3, 4 A subset of patient has a history of another malignancy particularly breast cancer. 5 Tubal carcinoma found with increased frequency in patient with germ line mutation involving BRCA-1 and BRCA-2 gene. Carriers of these mutations also have higher risk of developing of breast, ovarian and peritoneal carcinomas. Germ line mutation have been detected in 15 to 43% of patient of tubal carcinoma.5 Life time risk of developing of tubal carcinoma is 0.6% in BRCA mutation carriers 6 and 0.2% in the general population. 7 The fallopian tube has an indirect role in the pathogenesis of endometrioid and clear cell carcinomas of the endometrium and ovary. 8
For many decades it was assumed that HGSCs arise from the ovarian surface epithelium, but in 2001 this line of thinking was challenged by the identification of presumptive (HGSC) precursors—serous tubal intraepithelial carcinomas (STICs), and occult HGSCs in the fallopian tubes of patients with germ line BRCA1 mutations who were undergoing prophylactic surgery. 9 Detailed examination of prophylactic specimens in this patient population implicated the tubal fimbriae as the origin of HGSC. 10 Additional evidence of tubal origin of HGSC was provided by several subsequent studies showing STIC with invasive carcinoma confined to the fallopian tube in women without hereditary predisposition to ovarian cancer. 11 Consequently, a recent consensus statement on primary site assignment of tubo-ovarian HGSCs recommends assigning cases as tubal in origin if STIC or invasive mucosal carcinoma is identified in the fallopian tube. 12 Immunohistochemical staining demonstrates aberrant p53 protein expression (either diffuse nuclear overexpression or complete absence of staining) and an increased Ki-67 proliferation index in the lesional epithelium. 13, 14 Both BRCA1/2 mutation–positive and sporadic cases of non uterine HGSC have been shown to be associated with concomitant STIC, with matched pairs of STIC-HGSC harboring identical TP53 mutations15.
Some STIC may be diagnosed on H&E stained slide, but there is substantial inter observer variability among gynecologic pathologist. 15, 14 However morphology and immunohistochemistry shows improved reproducibility of the diagnosis. 14, 15 Immunohistochemical stain with p53 and Ki-67 is very much helpful for diagnosis of STIC. CK, p16, WT-1 and PAX-8 are also helpful for diagnosis. Serum CA125 is elevated in most patients although it may be normal in those with tubal intraepithelial carcinoma.3, 4
Serous tubal intraepithelial carcinoma (STIC) is a rare pathologic finding at the time of benign gynecologic surgery. Tubal carcinoma is discovered incidentally in a small subset of patients who undergo surgery for other non neoplastic gynecological disorder.4 It usually arises in the distal fimbriated end of the fallopian tube and likely represents a precursor lesion to high-grade pelvic and ovarian serous carcinoma. A detailed investigation of the entire length of the fallopian tube (including its fimbriae) found a high rate of concurrent STIC in patients with ovarian serous adenocarcinoma or peritoneal serous adenocarcinoma. We are presenting a patient who was unexpectedly found to have high grade mucosal serous carcinoma of fallopian tube along with serous tubal intraepithelial carcinoma (STIC) when tubectomy was done for ectopic tubal gestation. Diagnosis was done on the basis of histomorphological features and relevant immunohistochemistry.
Ectopic tubal gestation may be due to partial obstruction caused by intraluminal mucosal serous carcinoma of fallopian tube. Rare coexistence of a tubal ectopic pregnancy with tubal leiomyoma, adenofibroma and adenomatoid tumour were also reported in literature.16, 17, 18
The diagnosis, implications, management options and prevention strategies for STIC are also discussed here.
Materials and Methods
A 26yrs, female, with 7wks and 6 days of period of gestation presented in outpatient department of obs. & gynaecology with complain of pain abdomen. There was no history of bleeding P/V. She was Para 1+1. She had history of previous LSCS. She had also history of right sided ectopic pregnancy, for which right sided tubectomy was done two years back. Ultrasonography examination revealed normal sized uterus without any gestational sac or embryo in uterine cavity and no other abnormality was also detected. An echogenic lesion in left adnexal region with small anechogenic central mass was seen. Left sided tubal pregnancy was suspected. Emergency exploratory laparotomy with left sided salpingectomy was done.
O.T note revealed normal uterus, both ovaries healthy but ampullary area of left fallopian tube shows a dilatation (3cmx3cm) with rupture of tube with bleeding. Two units of blood were transfused in post operative period. Post operative period was otherwise uneventful.
Specimen of fallopian tube was sent for histopathological examination. Specimen received in histopathology department as product of conception in left fallopian tube.
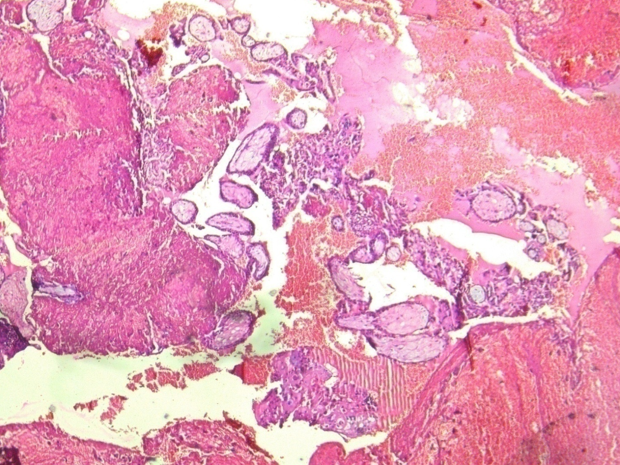
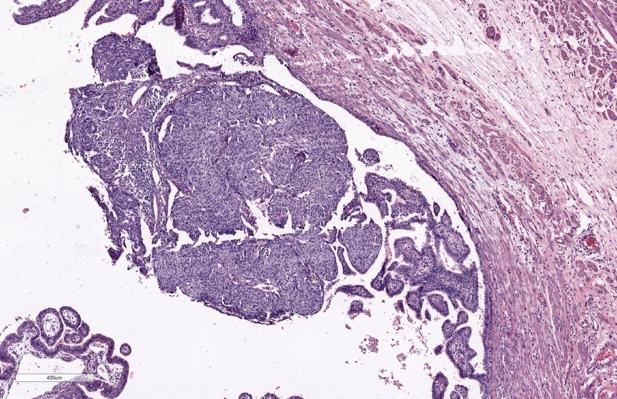
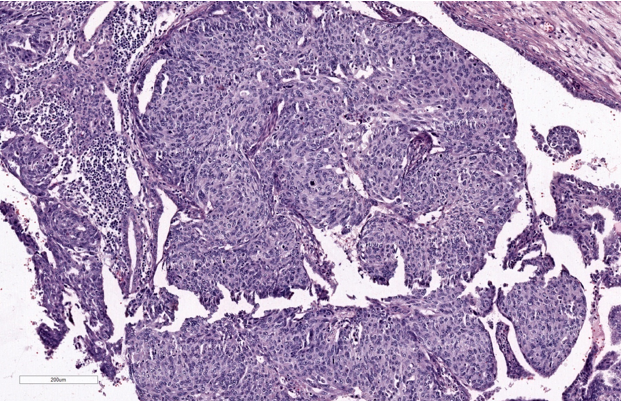
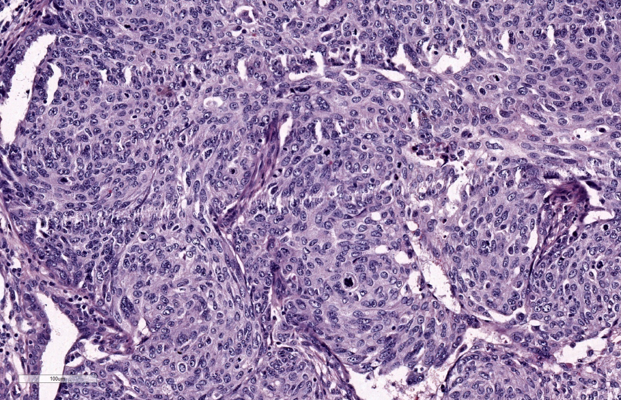
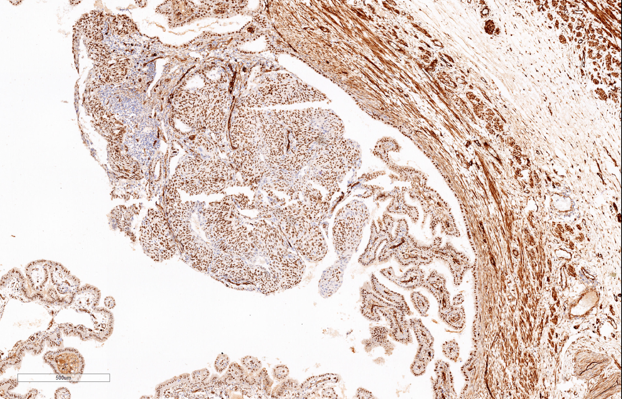
Gross examination of the specimen shows, dilated fallopian tube measuring 6cm in length and 3.5cm in maximum diameter. The outer surface was grey brown in colour. Cut section shows blood clot and brownish areas. Multiple representative sections were submitted for histopathological study. Later all the material was embedded for further study. Microscopic examination on H&E. stained slides show areas of dilated fallopian tube with multiple chorionic villi admixed with fibrin and blood clot. (Figure 1) Plical hyperplasia and muscular hypertrophy are also seen. Focal area also shows intraluminal atypical proliferation of the cells arising from tubal epithelium and which are arranged as sheets. Cells are non ciliated with loss of polarity, increased N/C ratio, rounded nuclei, course clumped chromatin, and conspicuous nucleoli. Increased mitotic activity and occasional atypical mitosis are seen. (Figure 2, Figure 3, Figure 4)
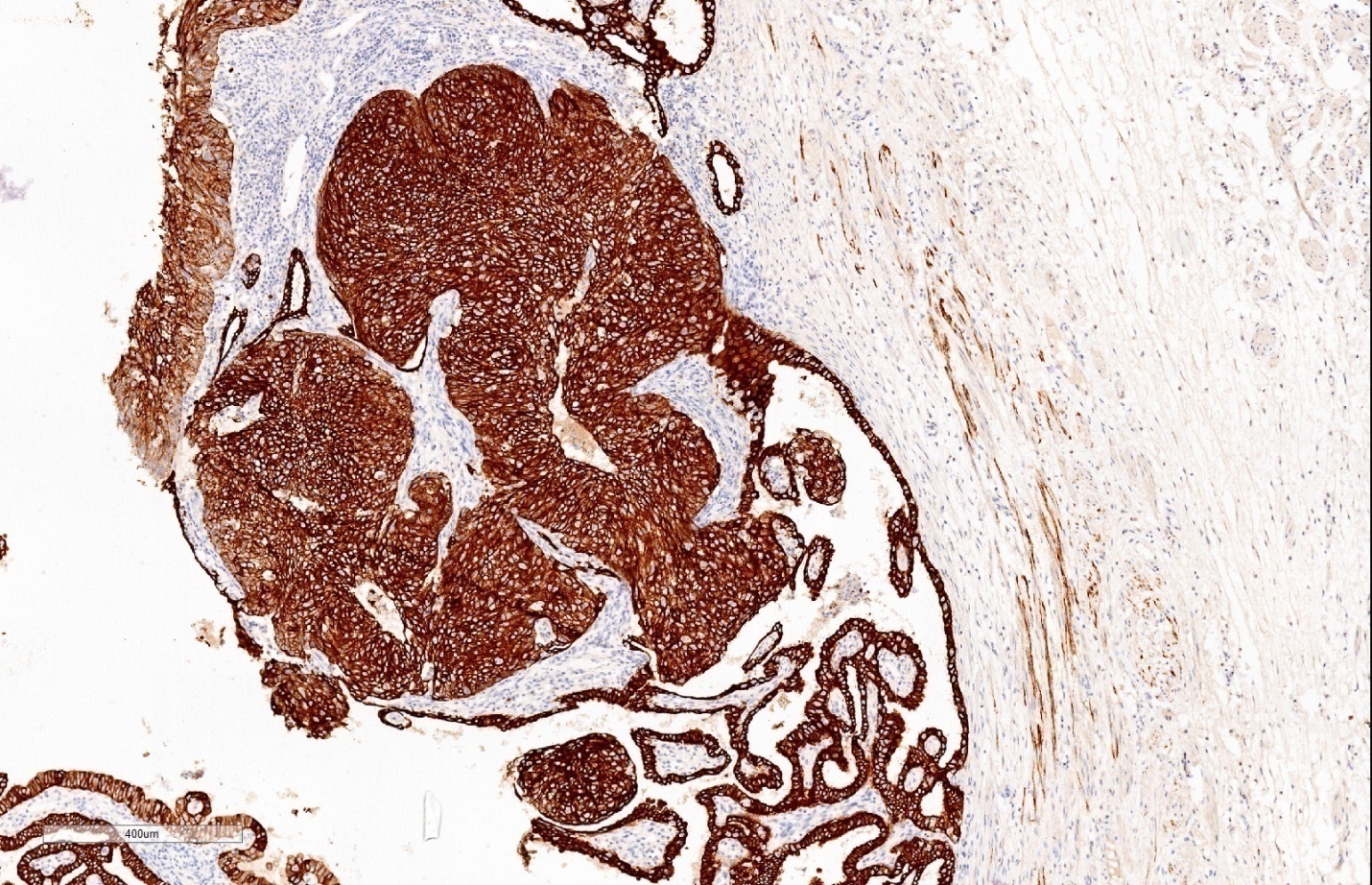
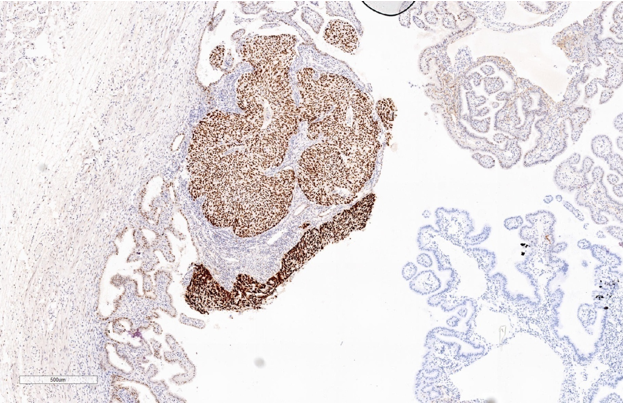
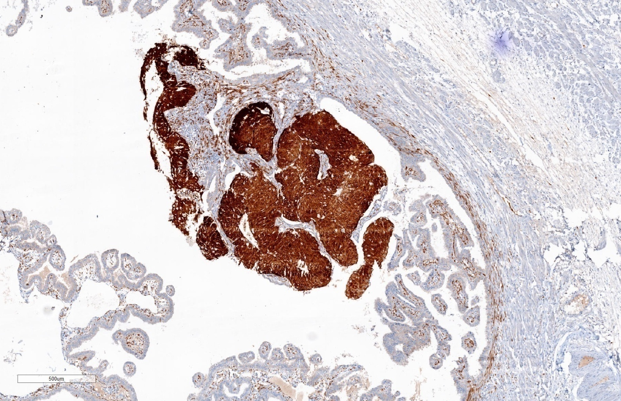
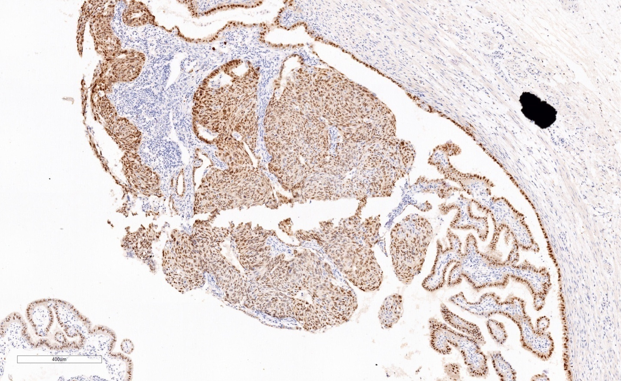
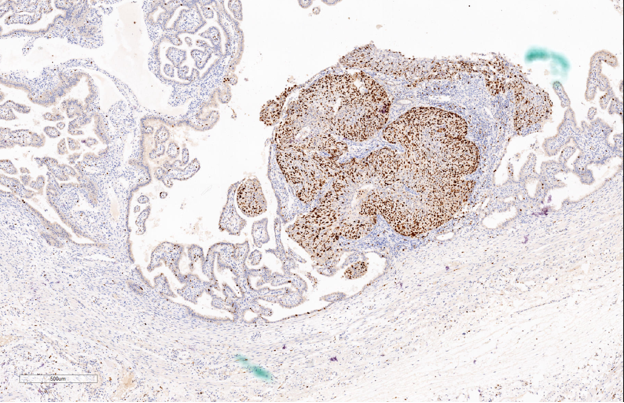
Immunohistochemistry study for CK, p53, p16, PAX-8, WT-1 and Ki-67 are advised on this section. Immunohistochemistry done on this section which are strongly and diffusely positive for Cytokeratin (AE1/AE3), p53 (Mutated phenotype), p16, PAX8, and WT1.(Figure 5, Figure 6, Figure 7, Figure 8, Figure 9). Immunostaining of Ki67 shows high proliferative index (Figure 10). Diagnosis of high grade mucosal serous carcinoma of fallopian tube in a case of ectopic tubal gestation was done.
Discussion
Primary carcinoma of fallopian tube is uncommon and it has been considered to account only 0.7 - 1.5% of all gynecological malignancies.1 Over half of tubal carcinomas are serous carcinoma.3, 4 The incidence of STIC has primarily been studied in patients with known BRCA mutations or a strong family history of breast or ovarian cancer and is estimated to be in the range of 0.6% to 6%.19
STIC is a pathologic finding of unclear clinical significance, but it appears to be a precursor lesion for high-grade pelvic (tubal, ovarian, or primary peritoneal) serous carcinoma usually arising in the distal fimbriated end of the fallopian tube.20
HGSCs have a strong association with BRCA mutations and almost ubiquitously harbor TP53 mutations.13 Pathologists and clinicians can thus benefit from a better understanding of tumor pathogenesis. The current prevailing theory is that HGSOCs arise from STICs of the fallopian tube. Microscopically, the epithelium of the fallopian tube consists of a mixture of secretory and ciliated cells. Secretory cells predominate in the isthmus of the tube, and ciliated cells are most conspicuous at the fimbriated end.21 Spectrum of entities originating from secretory cells have been described, including secretory/stem cell outgrowths (SCOUTs), p53 signatures, serous tubal epithelial proliferations or lesions of uncertain significance (STEP-US), and serous tubal intraepithelial carcinomas (STIC). STIC is earliest histologically identifiable lesion on the pathogenesis of fallopian tube carcinoma and it appears to arise from a putative precursor the p53 signature. This lesion is characterized histologically by normal tubal epithelium that shows strong and diffuse p53.22 Over half of the p53 signature harbor mutation in TP53.22 Both STIC and p53 signature appears to arise from SCOUTs which are defined as linear outgrowth of at least 30 consecutive secretary cells that may be associated with serous carcinogenesis.
On this spectrum of pathologies, STIC is the most morphologically atypical. It is characterized as secretory cell lesions with some degree of cellular depolarization with epithelial stratification, increased nuclear to cytoplasmic ratios, hyperchromasia, nuclear molding, prominent nucleoli, and increased mitotic activity.14 In addition to these morphologic criteria, TP53 mutations are present in 92% of STICs. The lesions therefore typically demonstrate strong and diffuse p53 immunohistochemical staining consistent with missense mutations. Less commonly, there is complete absence of staining due to nonsense mutations in TP53.13 Many normal tissues and tumors unassociated with TP53 abnormalities express p53 protein. Such staining is usually focal and weak and somewhat variable from area to area (referred to as “wild‐type” p53 staining). Typically in excess of 75% and sometimes almost all of the nuclei are intensely positive. It should also be appreciated that totally absent p53 staining (as stated, there is usually an inbuilt positive control with “wild‐type” staining of non neoplastic tissues) is also indicative of aberrant p53 immunoreactivity23 Diffuse intense nuclear immunoreactivity and totally absent staining (“all or nothing”) are aberrant patterns (“mutation‐type” staining) and in keeping with an underlying TP53 mutation while“wild‐type” staining is not.
STICs are not identifiable grossly. As such, the Sectioning and Extensively Examining the Fimbriated End (SEE-FIM) protocol was published in 2006 in an effort to detect STICs more reliably.24 The protocol calls for prophylactic salpingo-oophorectomy specimens to be submitted in their entirety. For the fallopian tubes, the distal two centimeters, including the fimbriae, are amputated and then longitudinally sectioned into four sections. The reminder of the fallopian tube is sectioned at 2 mm to 3 mm intervals.24
Immunohistochemistry has many important applications in the field of tubo ovarian neoplasia.25, 26 Some STIC may be diagnosed on H&E, but there is substantial interobserver variability among gynecologic pathologist. 14 However morphology and immunohistochemistry shows improved reproducibility of the diagnosis.14, 15
Morphologically, STICs are characterized by a proliferation of non ciliated epithelium showing nuclear stratification, marked nuclear pleomorphism, prominent nucleoli, and mitotic figures. Immunohistochemical stain with p53 and Ki67 very much helpful for diagnosis of STIC.
Immunohistochemical staining demonstrates aberrant p53 protein expression (either diffuse nuclear over expression or complete absence of staining) and an increased Ki-67 proliferation index in the lesional epithelium.13, 14 Both BRCA1/2 mutation–positive and sporadic cases of non uterine HGSC have been shown to be associated with concomitant STIC, with matched pairs of STIC-HGSC harboring identical TP53 mutations27, 28 p16 are sensitive and specific adjunct biomarkers that, when used with p53 and Ki-67, improve the diagnostic accuracy of STIC.29 p16 were expressed in the majority of p53-positive and p53-negative STICs and concomitant invasive HGSCs. Diffuse p16 positivity observed in STIC, but can be seen only focally in normal tubal epithelium. 30 Ki-67 cut off level of 10% in lesional epithelium has been suggested. 15 In one of the study mean Ki-67 labeling index was 72% (range 40-95%).31
Most tubo‐ovarian serous carcinomas exhibit diffuse nuclear positivity with WT1 while most uterine serous carcinomas are negative. However, there is some overlap in that a proportion of uterine serous carcinomas are WT1 positive (the percentage has varied between studies) and a small percentage of tubo‐ovarian high‐grade serous carcinomas are WT1 negative21, 32 WT-1 immunohistochemical expression was shown in majority of serous carcinomas of ovary and fallopian tube establishing as a highly sensitive as well as specific marker for tumors of mullerian differentiation.33, 34
HGSCs express protein markers characteristic of Mullerian epithelium (eg, PAX-8) and do not express calretinin, a mesothelial marker that is also expressed by the ovarian surface epithelium but not the fallopian tube epithelium.35
We have described here a patient who was unexpectedly found to have high grade mucosal serous carcinoma of fallopian tube along with serous intraepithelial carcinoma (STIC) when tubectomy was done for ectopic tubal gestation. Diagnosis was done in this case with the help of morphology and immunohistochemical stains. CK, p53, p16, WT-1 and PAX-8, showed strong and diffuse positivity & Ki-67 also showed high proliferative index.
BRCA germ line mutation study was advised, but in this case patient refused to undergo genetic testing. The knowledge of her BRCA status could affect which screening options she is offered for breast cancer surveillance and could also impact her family members. If she were positive for a BRCA germ line mutation, immediate BRCA testing could be implemented in her family members to either confirm their high-risk status or clear them of risk. For now, she continues to be evaluated every six months with pelvic exam and CA-125 level. Given the rarity of an STIC diagnosis, there are limited data on clinical outcomes or management strategies. As demonstrated by Wethington et al.36 the value of surgical staging may be low. It has been suggested that intraluminal mass without invasion qualify as neither stage -0 nor stage IA.37 Five year survival of stage 0 ranges from 75-91%.6, 38 Alvarado-Cabre et al. showed that sub categorization of stage-1 cases based on depth of invasion was prognostically significant.37 For women with only STIC in the absence of positive washings or evidence of malignant spread, empiric adjuvant chemotherapy similar to what would be recommended by National comprehensive Cancer Center Network (NCCN) guidelines for stage I ovarian or fallopian tube cancer could be given.39 However, given the possibility of adverse effects, a risk-benefit ratio should be performed between the physician and patient prior to initiating chemotherapy. More studies are needed assessing effective management strategies but will be limited by low numbers. So, possible management options include observation with annual physical examination and CA-125 estimation, surgical staging, or empiric chemotherapy. However, due to the lack of consensus regarding management options, referral to a gynecologic oncologist recommended.
Associated ectopic gestation may be due to partial obstruction caused by intraluminal mucosal serous carcinoma of fallopian tube. Rare coexistence of a tubal ectopic pregnancy with tubal leiomyoma, adenofibroma and adenomatoid tumour was also reported in literature.16, 17, 18 The close spatial relationship of two such lesions, suggests that a neoplastic lesion can interfere transportation of the fertilized ovum through the tube possibly via impaired contractile activity of myosalpinx and consequently cause the ectopic tubal pregnancy.16