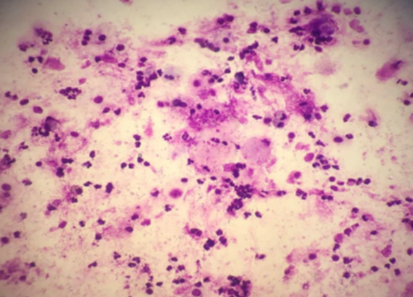
Introduction
Interstitial lung diseases (ILDs) refers to acute and chronic bilateral infiltrative lung diseases with variable degree of tissue inflammation and fibrosis when they occur in immunocompetent hosts without infection or neoplasm.1 ILDs exhibit similar clinical presentations, but a precise diagnosis is essential because the diseases' prognosis and courses of treatment vary. Proper clinical history, clinical examination, chest radiography findings, and lung biopsy are essential for differentiating between these illnesses. When radiologic imaging and lung biopsy provide inadequate information to diagnose interstitial lung disease (ILD), bronchoalveolar lavage (BAL), a safe and less invasive procedure, is utilized as an auxiliary diagnostic tool. 2 Although high resolution computed tomography (HRCT) is specific for diagnosing ILD, BAL can help narrow down the differential diagnosis in clinically suspect ILD even when HRCT is normal. 2
Furthermore, BAL differential cellular analysis may not be utilized enough in the diagnosis of ILD, despite its widespread use. Information derived from the BAL cell differentials with a lymphocytic, neutrophilic, eosinophilic and mixed cellular pattern can be used as an adjunct to diagnosis.3, 4 A BAL cell analysis with Lymphocytes >15% is termed as Lymphocytic cellular pattern which is most commonly seen in Sarcoidosis, Hypersensitive pneumonitis (HP) and connective tissue diseases. Likewise, Neutrophilic cellular pattern with neutrophils >3% is noticed in Idiopathic Pulmonary fibrosis, Acute Interstitial Pneumonia, Sarcoidosis (advanced) and Hypersensitive pneumonitis (acute), Similarly, Eosinophils >1% is identified as Eosinophilic cellular pattern which is seen in Chronic Eosinophlic Pneumonia and Allergic bronchopulmonary aspergillosis. An increase in more than one type of cells in BAL fluid represents Mixed pattern which is commonly seen in Cryptogenic organizing pneumonia, Idiopathic pulmonary fibrosis.3, 4, 5
BAL was utilized for many years as part of the workup for ILD, primarily in patients with idiopathic pulmonary fibrosis (IPF), hypersensitivity pneumonitis (HP), and sarcoidosis.5 But as high resolution computed tomography (HRCT) advances, this function appears to be declining. It is best to perform a BAL with a pre-procedural chest HRCT and differential cell count analysis when a patient is suspected of having ILD.6
Materials and Methods
We performed a descriptive cross-sectional study after getting ethical clearance (IRC/1673/019) in the Cytopathology Laboratory at B.P. Koirala Institute of Health Sciences (BPKIHS), Dharan for a period of one year (March 2020 to February 2021). Consecutive sampling technique was used. The subjects were chosen from the Out-patient Department, Department of Pulmonary Critical Care and Sleep Medicine, BPKIHS. A BAL was performed on a patient with suspected ILD, and clinical and laboratory data were evaluated. Using a bronchoscope, normal saline (100–300 ml in 3–5 aliquots) was inserted into the targeted region. The recovered BAL fluid was transported "fresh" at room temperature and was centrifuged in the cytopathology laboratory. Using centrifuged slides, at least 400 cells were cytologically examined after being stained with Giemsa and Papanicolaou. Well Informed consent was taken from all patients.
Results
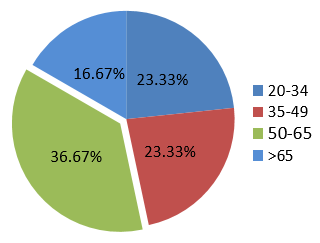
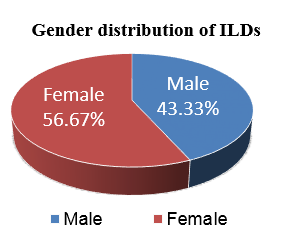
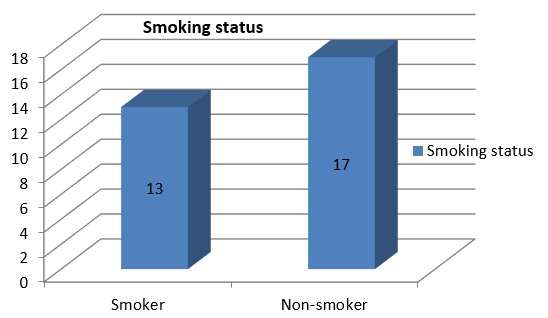
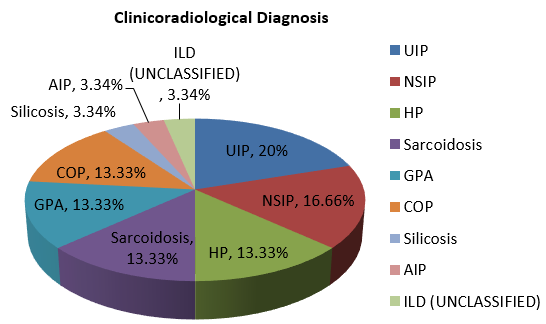
A total of thirty two cases of clinicoradiologically suspected ILDs were received for our investigation. Out of them, thirty cases were included in our study because, because one case proved insufficient, and another had been designated as possibly malignant. Age and gender distribution are depicted in Figure 2, Figure 3. ILD was more common in non-smokers which is shown in Figure 4, Figure 5. Out of total 30 cases with clinicoradiological suspicion, six cases (20%) of Usual Interstitial Pneumonia (UIP), five cases (16.66%) of Non-specific Interstitial Pneumonia, four cases (13.33%) each of Hypersensitivity Pneumonitis (HP), Granulomatous Polyangitis (GPA), Sarcoidosis, and Cryptogenic Organizing Pneumonia (COP) were received. Acute interstitial pneumonia (AIP) and silicosis each had one case (3.34%). Because subcategorization was not possible, one case (3.34%) was assigned an ILD-post-COVID status.
Among six cases that were suspicious of UIP clinicoradiology, three of them exhibited a neutrophilic cellular pattern, one each had mixed, lymphocytic, and normal cellular patterns. Out of five cases received as suspicious of NSIP clinicoradiologically, two of them had Neutrophilic cellular pattern, other two cases had normal cellular pattern and one case had Mixed cellular pattern. Among four cases of clinicoradiologically suspicoius HP, each one had Lymphocytic, Neutrophilic, Mixed and Normal cellular pattern. Similarly, among four cases having clinicoradiological suspicion of sarcoidosis, three cases presented with lymphocytic cellular pattern and one with mixed cellular pattern. This was followed by four cases of GPA, where each case presented with Lymphocytic, Neutrophilic, Mixed and Eosinophilic cellular pattern. Likewise, out of four cases of COP, three cases had Neutrophilic cellular pattern and one had Lymphocytic Cellular Pattern. Mixed cellular pattern was observed in one case received as AIP and the case received as ILD (unclassfied) had Normal cellular pattern.(Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11 )
Table 1
BAL differential counts in ILDs
Table 2
BAL differential count in suspected cases of Sarcoidosis
|
Various studies |
Macrophages (%) |
Lymphocytes (%) |
Neutrophils (%) |
Eosinophils (%) |
|
Present study Mean(Median) |
72(70) |
25.75(26.5) |
5.5(2.5) |
0.75(0) |
|
Efared et al.7 (mean) |
46.1 |
38.13 |
14.22 |
1.89 |
|
Lee W et al.2 (mean) |
54.40 |
43.77 |
1.39 |
0.34 |
|
L. Welker et al8 (median) |
- |
27 |
1 |
0 |
Table 3
BAL differential cell count in suspected cases of UIP
|
Various studies |
Macrophages (%) |
Lymphocytes (%) |
Neutrophils (%) |
Eosinophils (%) |
|
Present study Mean(median) |
77.5(79.5) |
12.33(7.5) |
9.66(6) |
0.33(0) |
|
Lee W et al.(mean)2 |
49.18 |
21.21 |
22.08 |
7.50 |
|
Nagai S et al. (mean)9 |
83 |
7.2 |
5.9 |
3.3 |
|
S. Veeraraghavan et. al10 (median) |
73 |
4 |
9 |
7 |
|
Kinder et. al11 (median) |
- |
8 |
6 |
2 |
|
Daniil et al12 (mean) |
76.8 |
8.4 |
9.6 |
5.8 |
Table 4
BAL differential count in suspected cases of NSIP
|
Various studies |
Macrophages (%) |
Lymphocytes (%) |
Neutrophils (%) |
Eosinophils (%) |
|
Present study Mean(Median) |
67.4 (73) |
11(10) |
21.4(4) |
0.2(0) |
|
S. Veeraraghavan et al.10 (median) |
71 |
5 |
9 |
7 |
|
Lee W et al.2 (Mean) |
40.67 |
43.54 |
8.81 |
6.96 |
|
Nagai et al.9 (Mean) |
51.8 |
40.0 |
2.5 |
5.7 |
|
Daniil et al.12 (Mean) |
79.3 |
9.3 |
7.8 |
3.2 |
Table 5
BAL differential count in suspected cases of HP
|
Various studies |
Macrophages (%) |
Lymphocytes (%) |
Neutrophils (%) |
Eosinophils (%) |
|
Present study Mean(median) |
73.5(77.5) |
13.5(14) |
13(7.5) |
0(0) |
|
Lee W et al2 (mean) |
55.31 |
19.92 |
15.54 |
8.88 |
|
Caillaud et al13 (mean) |
35.5 |
53.2 |
9.2 |
1.2 |
|
Chockalingam et al.14 (range) |
- |
26-55% |
5-20% |
- |
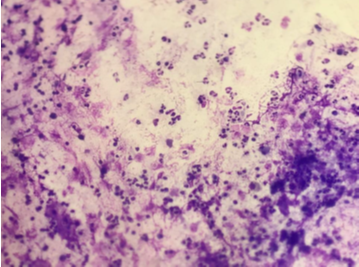
Figure 5
(Giemsa, 400X) Increased Lymphocytes count along with alveolar macrophages in suspected case of Sarcoidosis
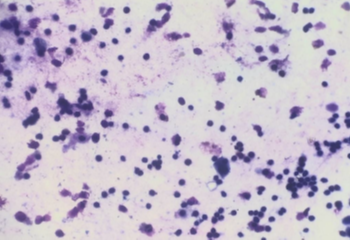
Figure 6
(Giemsa, 400X) Increased Lymphocytes count along with alveolar Macrophages in suspected case of UIP
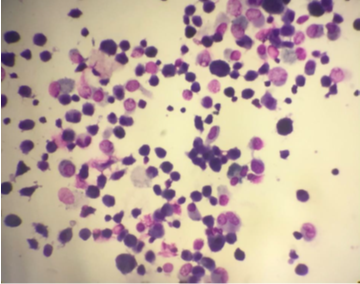
Figure 7
(Giemsa, 400X) Neutrophilic Cellular Pattern in suspected case of Hypersensitivity Pneumonitis
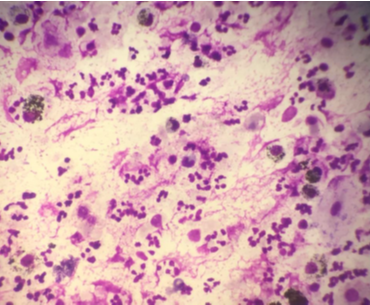
Figure 8
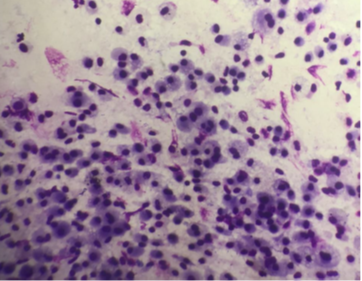
(Giemsa, 400X) Normal cellular pattern in suspected case of Non-specific Interstitial Pneumonia.
Discussion
Bronchoalveolar lavage (BAL) is a common and relatively safe procedure in the evaluation of lung disease as it allows for sampling of the lower respiratory tract. BAL differential cell counts have been shown to be indicative of particular lung disorders in a number of cases. BAL frequently offers useful diagnostic information when coupled with an adequate clinical history, physical examination, laboratory studies, and radiographic findings. 15
With a male to female ratio of 1:1.3 in our study, ILDs were found to be somewhat more common in females, which is consistent with a study by Duchemann et al.,16 that found a male to female ratio of 1:1.1. The male to female ratio in the study done by Coultas et al.17 was 1.1:1. Nonetheless, the distribution of cases between males and females was nearly equal.
According to our study, there was a slight predominance of non-smokers in 17 cases (56.67%) compared to 13 patients (43.33%) who had a positive smoking history. A study done by Duchemann et al.16 revealed that there were 482, 71, and 213 non-smokers, current smokers, and ex-smokers, respectively. The study conducted by Kelvin et al.18 found that a mere 20% of participants were smokers. Due to the preponderance of non-smokers, our analysis revealed consistency with both of these investigations.
BAL differential count in suspected cases of sarcoidosis
In our study, the mean percentages of macrophages, lymphocytes, neutrophils and eosinophils in the clinicoradiologically suspected cases of Sarcoidosis were 68%, 25.75%, 5.5%, and 0.75%, respectively. In BAL fluid cytology, three of the cases exhibited lymphocyte counts greater than 25%, which indicated a lymphocytic pattern. Similar results were observed in other studied as well that are enlisted in Table 2. The remaining one case exhibited a mixed cellular pattern, with 1% eosinophil, 21% lymphocyte, and 16% neutrophil that aligns with research conducted by Efared et al. 7 and Chockalingam et al. 14 which indicates that the reason for mixed celluar pattern was either superadded infection or the disease's subacute character.
BAL cellular count in suspected cases of usual interstitial pneumonia (UIP)
The mean percentages of macrophages, lymphocytes, neutrophils and eosinophils in our study were, respectively, 77.5%, 12.33%, 9.66%, and 0.33%. Comparably, the median percentages of macrophages, lymphocytes, neutrophils and eosinophils were 79.5%, 7.5%, 6% and 0% respectively. These findings supported the increased neutrophilic count found in various other studies which is shown in Table 3. 2, 11, 10, 9, 12 However, results of eosinophil count in our study are at odds with some of them which are mentioned in the same table. 2, 10, 9
BAL differential count in suspected cases of Non-specific Interstitial Pneumonia (NSIP)
The mean percentage of neutrophils, lymphocytes, macrophages, and eosinophils in our study revealed results that were consistent with those of some research that found higher neutrophil counts. 10, 12 However, several other studies indicate a higher lymphocyte count, which is in odds with our findings. Table 4. shows the comparison table between our study and other research projects. 2, 10, 9, 12 According to K.C. Meyer et al. 4, fibrotic NSIP exhibits varied counts of lymphocytes, neutrophils, and eosinophils, while cellular NSIP is associated with an elevated neutrophil count. This could be the cause of the variations in findings between different studies.
BAL differential count in suspected cases of Hypersensitivity Pneumonitis (HP)
BAL cellular examination of four suspected HP cases showed one each of mixed cellular (with lymphocyte 16% and neutrophil13%), neutrophilic, lymphocytic, and normal cellular patterns. One case with Lymphocytic pattern supports the findings in comparision to most of the studies found in literature. 19, 20, 13 According to Abdo and Kebbe et al.,5 a neutrophil cellular pattern may be observed in acute HP. This could explain one of the neutrophilic pattern and another mixed cellular pattern cases in our study.5, 14 Normal cellular pattern, however, does not match findings from these investigations. This could therefore assist clinicians in considering different diagnoses. Table 5 displays the comparison table between our study and others.
BAL differential count in suspected cases of cryptogenic organizing pneumonia (COP)
The BAL results of COP, according to Davidson et al. 15 and Cordier JF et al. 21, are nonspecific, showing a mixed cellular pattern with a varied rise in neutrophils, eosinophils, and lymphocytes. Acute fulminant cases are often Neutrophilic and more chronic cases are lymphocytic. Three of the four suspected cases of COP that were included in our analysis exhibited neutrophilic cellular pattern, while one case had lymphocytic cellular pattern.
BAL differential count in suspected cases of granulomatous polyangitis (GPA)
The mean percentages of macrophages, lymphocytes, neutrophils and eosinophils in our study are 46%, 40.5%, 19%, and 0%, respectively. Out of four cases of suspected GPA, each one had different cellular pattern that was characterized as lymphocytic, neutrophilic, mixed (lymphocyte+neutrophil), and eosinophilic (with 25% eosinophil), respectively. In our study, among four cases, three cases (Neutrophilic, Mixed and Lymphocytic) were in concordance with other studies. 15, 22, 23 Kebbe et al. 5, Chockalingam et al.,14 Cotabel et al.,24 and Davidson et al.15 state that an eosinophil count of more than 25% is almost exclusively indicative of acute or chronic eosinophilic pneumonia. Therefore, it helps rule out the possibility of GPA in case with > 25% eosinophils and directs the physician to consider other possible causes.
Conclusion
Interstitial lung diseases remain one of the most challenging medical conditions which require multidisciplinary approach. In conjunction with clinical and HRCT findings, bronchoalveolar lavage (BAL) may be a crucial test that helps the physician confirm a diagnosis or restrict the differential diagnosis in suspected cases of ILDs. Additionally, BAL aids in the prediction of the acute or chronic nature of diseases and provides information on the state of superadded infections to aid in appropriate management.