- Visibility 77 Views
- Downloads 16 Downloads
- DOI 10.18231/j.achr.2024.049
-
CrossMark
- Citation
Soft tissue myoepithelioma arising over the scalp: Rare case report
- Author Details:
-
Seema Bijjaragi
-
G.V. Neethu
-
Vardendra Kulkarni
-
H.R. Chandrashekar
-
Apurva Shrivastava *
Introduction
Myoepithelial tumours in skin and soft tissue are uncommon but have been increasingly characterized over the past decade. Men and women are equally affected across all age groups and lesions arise most frequently on the extremities and limb girdles.[1] Myoepithelial tumours of soft tissue are categorised as tumours of uncertain differentiation by the latest WHO Classification of Tumours 5thed-Soft Tissue and Bone Tumours. Approximately 20% of cases occur in paediatric patients, in whom they are frequently malignant.[1] Myoepithelial neoplasms of soft tissue show a wide range of cytologic and architectural features both within a given lesion and between different tumours. [1]
These tumours are often well-circumscribed grossly, however, both benign and malignant tumours are unencapsulated and may show infiltrative margins. [1] Tumours are typically characterized by multinodular/ lobular growth of spindled, ovoid, or epithelioid cells arranged in variable growth patterns, such as reticular, trabecular, nested, and solid; embedded in a variably prominent myxoid, hyalinized or chondroid stroma.[1] The cytoplasm of individual cell ranges from eosinophilic to clear. Occasional other morphologic appearances include tumour cells with copious vacuolated cytoplasm (formerly identified in so-called “parachordoma”), plasmacytoid cells with densely eosinophilic cytoplasm (“hyaline bodies”), or rhabdoid morphology.[2] Similar to pleomorphic adenoma/mixed tumour of the salivary gland, heterologous differentiation occurs in up to 15 % of cases (most frequently cartilaginous[1] and/or osseous.[3], [4], [5], [6] and less commonly squamous[3] or adipocytic.[3], [4], [5], [6]
Myoepithelial tumours of soft tissue (as well as in those of skin and bone) commonly harbour EWSR1 gene rearrangements in half of the soft tissue lesions tested, with the common fusion partners POU5F1 and PBX1 identified in 16% of cases each.[7] Rare fusion partners include ZNF444, KLF17, ATF1, and PBX3, and occasional cases show alternative FUS gene rearrangements with similar partner genes.[7], [8], [9], [10] These studies have suggested some associations between genotype and phenotype. Most EWSR1-POU5F1-positive tumours present in deep soft tissues of the extremities in young patients and are composed of nests of epithelioid cells with clear cytoplasm; a subset of EWSFH-PBX1-positive tumours showed a deceptively bland and sclerotic appearance.[7] Myoepithelial carcinomas show nuclei with appreciable atypia and readily identifiable nucleoli. Subset of myoepithelial carcinomas have homozygous deletions of SMARCB1.[11] Mixed tumours with ductal differentiation have PLAG1 gene rearrangements (occasionally with LIFR as the fusion partner).[12], [13] The presence of rearrangements of PLAG1 and EWSFI1, respectively, in salivary gland pleomorphic adenoma and myoepithelial carcinoma emphasizes that soft tissue myoepithelial neoplasms are genetically related to their salivary gland counterparts.[14], [15]
Case Report
Clinical history
A 36-year-old male patient presented to surgery OPD with complaints of a gradually increasing painless swelling over the scalp for 5 years. Clinical examination showed a well demarcated, irregular, soft to firm, non-compressible and non-reducible swelling over left side of scalp measuring 11x3cm. No signs of inflammation/ pulsations/ punctum over the swelling. Skin over the swelling appeared normal.
Pre operative diagnosis of venous malformation or sebaceous cyst was made. Radiological assessment was not done. Mass was excised keeping the capsule intact and the specimen was sent for histopathological assessment.
Gross Examination
A fat attached grey-white to grey-brown soft tissue mass measuring 10x2.5x1.5cm noted. External surface was congested. Cut surface was grey-white to grey-brown showing solid-cystic areas with thick and thin walls. Haemorrhagic fluid drained from cystic spaces.
Microscopic examination
Multiple sections studied from haematoxylin and eosin stained solid cystic areas of soft tissue mass showed a well encapsulated benign neoplastic tumour ([Figure 1], [Figure 2]) composed of cells arranged predominantly in nests and trabeculae ([Figure 3]). The nests and trabeculae were separated by fibro-myxoid stroma with focal areas showing hyalinization. Tumour cells were large, spindled to epithelioid with eosinophilic to clear cytoplasm and uniform nucleus. Few cells showed prominent cytoplasmic vacuolations. No mitosis/necrosis/atypia seen. Impression–Features suggestive of Soft tissue Myoepithelioma (Scalp).
Immunohistochemistry showed diffuse strong positivity for S-100 & myogenic marker Calponin.
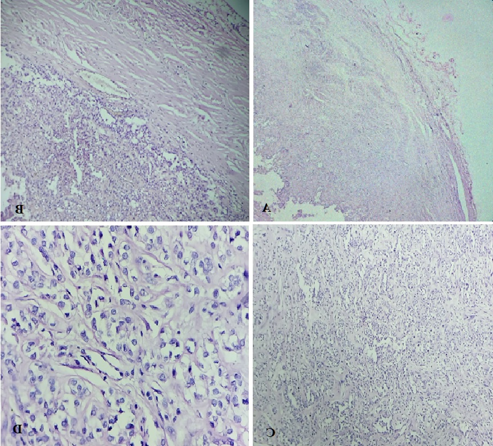
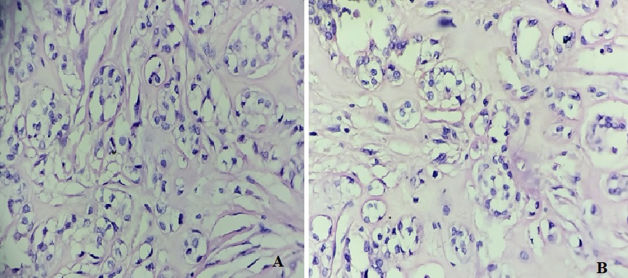
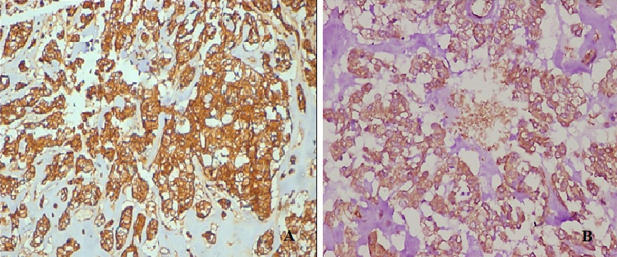
Discussion
Myoepithelial cells are usually found in glandular epithelium in sweat, mammary, lacrimal, and salivary glands. They constitute the basal cell layer of the secretory tubules. The myoepithelial neoplasm is a well-known entity in salivary gland; the extra-salivary counterpart has been described in the breast and retroperitoneum. In the skin, myoepithelioma has been recently recognized, which belongs to the cutaneous mixed tumours, also called chondroid syringoma. Three pathological variants of cutaneous mixed tumours are described. The most frequent apocrine variant is characterized by elongated branching tubular structures in a myxoid stroma. The eccrine variant presents small, round ductal structures in a myxoid or chondromyxoid stroma. Myoepithelioma, the rarest entity, represents a monophasic variant composed of purely myoepithelial cells. Myoepithelioma of the subcutaneous skin is extremely rare. [16]
The essential criteria laid down by the latest WHO Classification of Tumours 5thed-Soft Tissue and Bone Tumours to diagnose myoepithelial tumours includes; tumour cells arranged in trabecular, reticular, nested, and/or solid growth of variably spindled or epithelioid cells with a frequent myxoid or hyalinized stroma; mixed tumours in addition show ductal differentiation; myoepithelial carcinomas show increased cytological atypia and mitotic activity; positivity for EMA/keratin and S100, SOX10, or GFAP. Other desirable criteria in selected cases include EWSFI1 rearrangements confirmed using FISH technique.
Immunohistochemistry is necessary to confirm myoepithelial differentiation. Expression of myoepithelial markers is often variable in these tumours. Tumour cells express broad spectrum keratins and S100.[4] Other frequently positive IHC markers are EMA (-70%), GFAP (-50%), and SOX10 (-80%; expression is lower in myoepithelial carcinomas).[4] p63 is positive in a subset of tumours.[4]
Expression of myogenic markers is variable, with calponin being most frequently (90%) expressed. Other myogenic markers include SMA(60%) and desmin(0-20%).[4] PLAG1 is positive in mixed tumours, correlating with PLAG1 gene rearrangement.12 Loss of expression of SMARCB1 (INI1) is observed in a subset of myoepithelial carcinomas.[17]
Majority of the myoepithelial tumours of soft tissue show benign and have an indolent behaviour. Myoepithelial carcinoma is characterized by the presence of moderate to severe nuclear atypia with discernible nucleoli. [4] Histologically benign tumours have a 20% risk of recurrence, but they rarely metastasize, whereas myoepithelial carcinomas recur and metastasize in 40-50% of cases.[4] Common sites of metastasis include lungs, lymph nodes, bone, and soft tissue.[4]
Conclusion
In summary, this case report highlights the spectrum of myoepithelial tumours of soft tissue. Most of the soft tissue myoepithelial tumours are pure myoepitheliomas, whereas only 20% display ductal differentiation and hence can be classified as mixed tumours. Most morphologically benign or low-grade myoepithelial neoplasms of soft tissue behave in a benign fashion, with a low, but unpredictable, risk for local recurrence (approximately 20%). Soft tissue myoepithelial tumours with at least moderate cytologic atypia behave clinically malignant. [4]
Thus, careful, long-term follow-up is required because of the high recurrence rate and the rare risk of malignant transformation. [16]
Source of funding
None.
Conflict of interest
None.
References
- VY Jo, CD Fletcher. Myoepithelial neoplasms of soft tissue: an updated review of the clinicopathologic, immunophenotypic, and genetic features. Head Neck Pathol 2015. [Google Scholar]
- K Thway, N Bown, A Miah, R Turner, C Fisher. Rhabdoid Variant of Myoepithelial Carcinoma, with EWSR1 Rearrangement: Expanding the Spectrum of EWSR1-Rearranged Myoepithelial Tumors. Head Neck Pathol 2015. [Google Scholar]
- SE Kilpatrick, MG Hitchcock, MD Kraus, E Calonje, CD Fletcher. Mixed tumors and myoepitheliomas of soft tissue: a clinicopathologic study of 19 cases with a unifying concept. Am J Surg Pathol 1997. [Google Scholar]
- JL Hornick, CD Fletcher. Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol 2003. [Google Scholar]
- BC Gleason, CD Fletcher. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol 2007. [Google Scholar]
- VY Jo, CR Antonescu, L Zhang, P Dal Cin, JL Hornick, CD Fletcher. Cutaneous syncytial myoepithelioma: clinicopathologic characterization in a series of 38 cases. Am J Surg Pathol 2013. [Google Scholar]
- CR Antonescu, L Zhang, NE Chang, BR Pawel, W Travis, N Katabi. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer 2010. [Google Scholar]
- N P Agaram, HW Chen, L Zhang, YS Sung, D Panicek, JH Healey. EWSR1-PBX3: a novel gene fusion in myoepithelial tumors. Genes Chromosom Cancer 2015. [Google Scholar]
- U Flucke, T Mentzel, MA Verdijk, PJ Slootweg, DH Creytens, AJH Suurmeijer. EWSR1-ATF1 chimeric transcript in a myoepithelial tumor of soft tissue: a case report. Hum Pathol 2012. [Google Scholar]
- SC Huang, HW Chen, L Zhang, YS Sung, NP Agaram, M Davis. Novel FUS-KLF17 and EWSR1-KLF17 fusions in myoepithelial tumors. Genes Chromosom Cancer 2015. [Google Scholar]
- FL Loarer, F Zhang, CD Fletcher, A Ribeiro, S Singer, A Italiano. Consistent SMARCB1 homozygous deletions in epithelioid sarcoma and in a subset of myoepithelial carcinomas can be reliably detected by FISH in archival material. Genes Chromosomes Cancer 2014. [Google Scholar]
- A Bahrami, JD Dalton, JF Krane. A subset of cutaneous and soft tissue mixed tumors are genetically linked to their salivary gland counterpart. Genes Chromosomes Cancer 2012. [Google Scholar]
- CR Antonescu, L Zhang, SY Shao, JM Mosquera, I Weinreb, N Katabi. Frequent PLAG1 gene rearrangements in skin and soft tissue myoepithelioma with ductal differentiation. Genes Chromosomes Cancer 2013. [Google Scholar]
- C Martins, I Fonseca, L Roque, T Pereira,, C Ribeiro, J Bullerdiek. PLAG1 gene alterations in salivary gland pleomorphic adenoma and carcinoma ex-pleomorphic adenoma: a combined study using chromosome banding, in situ hybridization and immunocytochemistry. Mod Pathol 2005. [Google Scholar]
- A Skalova, I Weinreb, M Hyrcza, RHW Simpson, J Laco, A Agaimy. Clear cell myoepithelial carcinoma of salivary glands showing EWSR1 rearrangement: molecular analysis of 94 salivary gland carcinomas with prominent clear cell component. Am J Surg Pathol 2015. [Google Scholar]
- N Kadlub, E Galiani, S Fraitag, S Boudjema, MP Vazquez, A Coulomb. Soft tissue myoepithelioma of the scalp in a 11-year-old girl: a challenging diagnosis. Pediatr Dermatol 2012. [Google Scholar]
- JL Hornick, P Dal Cin, CD Fletcher. Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol 2009. [Google Scholar]
